
Note: If you aren't sure about structural isomerism, it might be worth reading about it before you go on with this page. Structural isomerism is not a form of stereoisomerism, and is dealt with on a separate page.

Where the atoms making up the various isomers are joined up in a different order, this is known as structural isomerism. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. This page explains what stereoisomers are and how you recognise the possibility of optical isomers in a molecule. Retrieved from: en.wikipedia.Optical isomerism is a form of stereoisomerism.
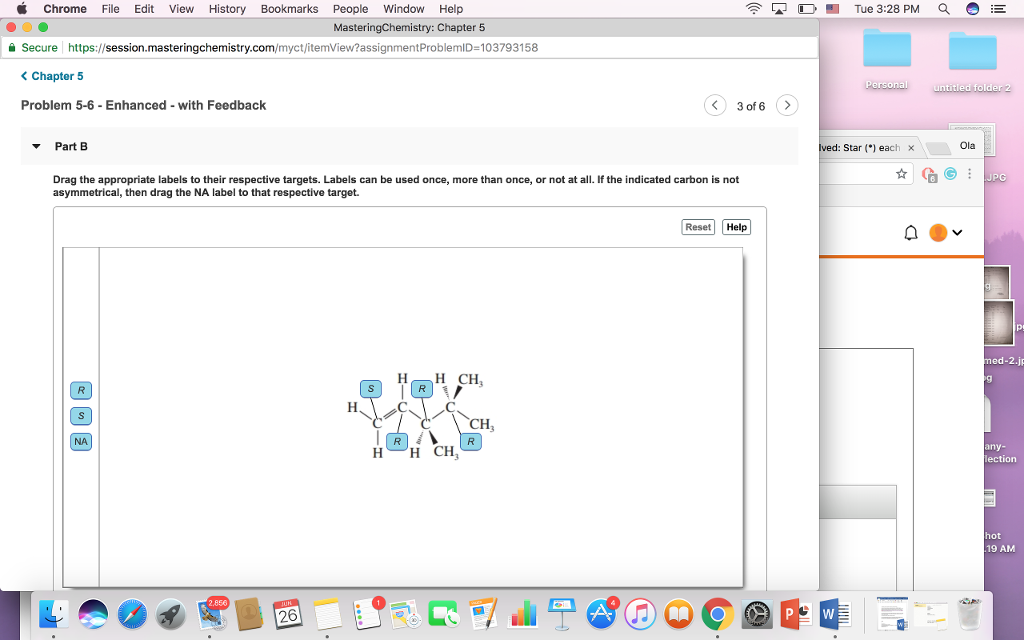
#Asymmetric carbon atom free#
Wikipedia, The Free Encyclopedia (2017).Definition of asymmetric carbon atom (s.f.).What is an Asymmetric Carbon? - Definition, Identification & Examples. Asymmetric Carbon, Sterioisomer and Epimer (s.f.).One of the asymmetric carbons is attached to the -OH group, and the remaining asymmetric carbon is bonded to the nitrogen atom. This nasal decongestant consists of two atoms of asymmetric carbons, that is, two formations whose center is given by the carbon atom, which, in turn, joins four different chemical elements together. Likewise, asymmetric carbons are also present in drugs, as is the case of pseudoephedrine (C 10 H fifteen NO), a medication used in the treatment of nasal congestion and pressure in the sinuses. They also occur in the ethyl groups, as for example in the structure of -CH2CH3, -OH, -CH2CH2CH3, -CH3, and -CH2NH3. This type of structures is common in organic compounds such as carbohydrates, for example. These structures have identical physical and chemical properties, they only differ in their optical activity, that is, the response they present to polarized light. In addition, each structure is called the enantiomer or optical isomer. That is, both images (real object versus reflection) are asymmetric with each other.Ĭonsequently, if you have a pair of structures with asymmetric carbons, and each of its elements are equal, both structures can not be superimposed on each other. Quilarity is the property that objects have not to overlap with the image that reflects in a mirror. If a chemical compound has two or more asymmetric carbons, the presence of quilarity in the total structure is induced. If an asymmetric structure is attached twice to a carbon atom, the latter could not be asymmetric. If the element were attached to the carbon by a double or triple bond, then the carbon would no longer be asymmetric. Each element must be linked to the asymmetric carbon through a single bond. In addition, certain conditions must be fulfilled in the constitution of the links, which are detailed below: The fact that the elements do not repeat themselves gives this formation the connotation of asymmetric or chiral. This configuration is similar to a star: the asymmetric carbon functions as the core of the structure, and the rest of the components start from it to form the respective branches of the structure. Characteristics of an asymmetric carbonĪsymmetric carbons are tetrahedral carbons that are connected to four different elements. This type of formations is very common in organic compounds, such as, for example, glyceraldehyde, a simple sugar obtained as a product of the process of photosynthesis in plants.


 0 kommentar(er)
0 kommentar(er)
